Chemoresponsive Liquid Crystal Research Database
This website presents key results of our team’s efforts to accelerate the design of chemoresponsive liquid crystalline systems that respond to targeted analytes, such as organophosphonates (e.g. DMMP), O3, Cl2, and formaldehyde. Our approach is to design of chemoresponsive liquid crystals iteratively by integrating work performed (Figure 1) under the direction of Profs. Manos Mavrikakis (quantum chemical modeling, UW-Madison), Robert J. Twieg (organic synthesis, Kent State University), and Nicholas L. Abbott (experimental physico-chemical evaluation, Cornell University). Through the tight and iterative coupling of computation, synthesis and characterization, we seek to overcome the slow, laborious and numerous experiments required previously to design chemoresponsive systems based on liquid crystals. Our work is supported by the National Science Foundation Division of Materials Research as part of the program called Designing Materials to Revolutionize and Engineer our Future (DMREF) under grant DMR-1435195 and DMR-1921696 and the Army Research Office as part of the Small Business Technology Transfer (STTR) program under grant W911NF-14-1-0140.
Figure 1. Our research team and its organizational setup.
Introduction to Liquid Crystals
Liquid crystals (LCs) are a phase of matter with properties intermediate between crystalline solids and isotropic liquids. The molecules in a nematic LC phase flow in response to shear like a fluid but retain a degree of long-range orientational order akin to a crystalline solid (Figure 2a). Liquid crystalline properties often stem from the ellipsoid-like structure of the molecules; the most often investigated LC is 4‐‐4′pentylbiphenyl (5CB, Figure 2b). The preferential orientation of molecules within a LC, termed the director (Figure 2c), can be influenced by the introduction of interactions between mesogens (molecules that form LCs) and chemically functionalized interfaces.
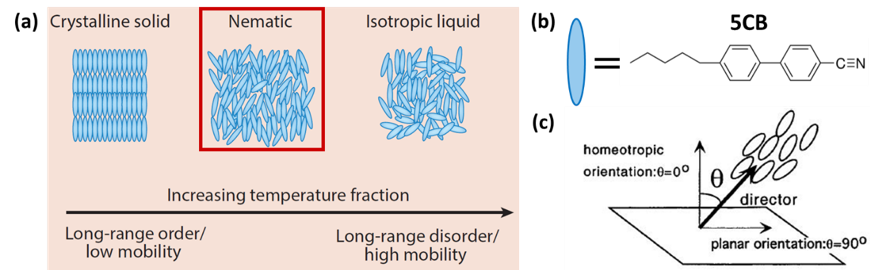
Figure 2. (a) Description of liquid crystalline phase. (b) 4‐cyano‐4′‐pentylbiphenyl is a widely investigated LC. (c) Definition of LC director near a surface.
Introduction to Chemoresponsive Liquid Crystals
The orientation assumed by a LC (i.e., orientation of the director characterizing the orientation of the LC long range order) near a surface is determined largely by the interactions of the LC molecules (mesogens) with the surface (Figure 3, left); e.g., the nitrile group of 5CB can bind to metal cations of metal salt surfaces. The binding of one surface monolayer of mesogens to metal cations on a surface can dictate the perpendicular orientation of a film of LC that has a thickness corresponding to hundreds of thousands of mesogens (homeotropic orientation, Figure 2c). The homeotropic orientation of the bulk LC film can be characterized by polarized light microscopy (the sample is dark under crossed polars). If we introduce an analyte that binds to the metal cation stronger than the mesogen, the analyte displaces the mesogen from its binding site on the surface (Figure 3, right) and the LC film transitions to a planar/tilted orientation (Figure 2c; the sample is bright under crossed polars). We use the readily quantified optical transition from dark to bright to detect the presence of targeted analytes. The video shows an optical transition of a LC film of 5CB supported on a surface presenting Al(ClO4)3 metal salt in the presence of 10 ppm of the nerve agent simulant called dimethylmethylphosphonate (DMMP) (note that the video is 20x faster than normal speed).
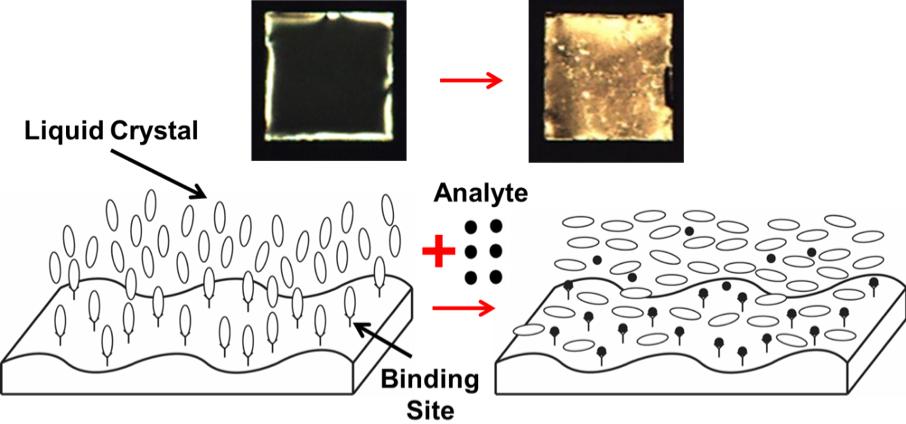
Figure 3. Detection of analytes with chemoresponsive liquid crystals.
.
Overview of Results
Our results are organized in terms of Generations. The term Generation is used to refer to the level of the calculation that is used to guide the experimental work. We performed several Cycles of computational chemistry-based predictions, syntheses and property measurements within each Generation of calculation. As the comparisons between experiment and computational predictions are performed, we are evolving the computational chemistry methodology to the next Generation with the goal of improving the predictive capability of the calculations and thus the speed and accuracy of the methodology for an increasingly efficient design of chemoresponsive LC systems.
Brief description of each generation:
-
Generation 1: Metal salts modeled by charged cation with implicit solvent model
-
Generation 2: Metal salts modeled by charged cation with explicit solvent model
-
Generation 3: Metal salts modeled by the neutral anion model
-
Generation 4: Metal surfaces modeled by periodic surface models
-
Generation 5: Metal oxide surfaces modeled by periodic surface models
In this website, Generation 1 calculations and experiments have treated the influence of solvent (e.g. EtOH or H2O) used in the synthesis of the salt surface, implicitly through the effective charge on the cation. Generation 2 calculations and experiments include explicit inclusion of the solvent molecules in the coordinative interaction between the metal ions, mesogens and the targeted analytes. Generation 3 calculations include the specific anions interacting with the metal cations, the targeted analytes, and the mesogens. Additional details are presented below.
While initial calculations used Generation 1, we now largely use Generation 3 calculations to guide the experimental work on metal salt surfaces. Feedback from experiments continues to improve the models within Generation 3. As detailed below, the predictive capability of Generation 3 computational methods is evidenced by computationally-driven designs of mesogen-metal salt interactions, which were subsequently validated via experimental synthesis and physico-chemical characterization. As a new direction, we also study mesogen-metal and mesogen-metal-oxide interactions that we summarize under Generation 4 and Generation 5, respectively.
We publically disclose our results on this website as soon as the work is published and/or relevant patents are filed. For each generation of calculations, we have webpages for: (i) mesogens, which provide the calculated binding strength of mesogens to metals cations, (ii) analytes, which provide the calculated binding strength of analytes to metals cations, and (iii) analyte-analysis webpages, which provide the calculated binding strength difference between mesogens and analytes to metal salts (displacement energy), together with important experimental characterization information. We also have a built-in search engine to quickly search within analyte-analysis pages and find optimal mesogen-metal salt combinations to detect targeted analytes. For further computational and experimental details, we direct the visitor to the following representative publications:
T. Szilvasi, H. Yu, J. Gold, N. Bao, T. Wolter, R. Twieg, N. L. Abbott, and M. Mavrikakis, "Coupling the Chemical Reactivity of Bimetallic Surfaces to the Orientations of Liquid Crystals," Materials Horizons, 2021, 8, 2050. 10.1039/D1MH00035G
N. Bao, J. I. Gold, T. Szilvási, H. Yu, R. J. Twieg, M. Mavrikakis, and N. L. Abbott, "Designing chemically selective liquid crystalline materials that respond to oxidizing gases," Journal of Materials Chemistry C, 2021, 9, 6507. 10.1039/D1TC00544H
H. Yu, K. Wang, T. Szilvási, K. Nayani, N. Bao, R. J. Twieg, M. Mavrikakis, and N. L. Abbott, "Design of Chemoresponsive Soft Matter Using Hydrogen-Bonded Liquid Crystals" Materials, 2021, 14, 1055. 10.3390/ma14051055
K. Wang, M. S. Rahman, T. Szilvási, J. I. Gold, N. Bao, H. Yu, N. L. Abbott, M. Mavrikakis, and R. J. Twieg, "Influence of multifluorophenyloxy terminus on the mesomorphism of the alkoxy and alkyl cyanobiphenyl compounds in search of new ambient nematic liquid crystals and mixtures," Liquid Crystals, 2020, 48, 672. 10.1080/02678292.2020.1810792
J. I. Gold, T. Szilvási, N. L. Abbott, M. Mavrikakis, “Binding of Organophosphorus Nerve Agents and Their Simulants to Metal Salts,” ACS Applied Materials & Interfaces, 2020, 12, 30941 . 10.1021/acsami.0c05777
K. Wang, T. Szilvási, J. I. Gold, H. Yu, N. Bao, P. Rai, M. Mavrikakis, N. L. Abbott, R. J. Twieg, “New Room Temperature Nematogens by Cyano Tail Termination of Alkoxy and Alkylcyanobiphenyls and Their Anchoring Behavior on Metal Salt-decorated Surface,” Liquid Crystals, 2020, 47, 540. 10.1080/02678292.2019.1662116
K. Wang, P. Rai, A. Fernando, T. Szilvási, H. Yu, N. L. Abbott, M. Mavrikakis, R. J. Twieg, “Synthesis and Properties of Fluorine Tail-terminated Cyanobiphenyls and Terphenyls for Chemoresponsive Liquid Crystals,” Liquid Crystals, 2020, 47, 3. 10.1080/02678292.2019.1616228
H. Yu†, T. Szilvási†, K. Wang, J. I. Gold, N. Bao, R. J. Twieg, M. Mavrikakis, N. L. Abbott, “Amplification of Elementary Surface Reaction Steps on Transition Metal Surfaces Using Liquid Crystals: Dissociative Adsorption and Dehydrogenation,” Journal of the American Chemical Society, 2019, 141, 16003. 10.1021/jacs.9b08057
K. Wang, M. Jirka, P. Rai, R. J. Twieg, T. Szilvási, H. Yu, N. L. Abbott, M. Mavrikakis, “Synthesis and properties of hydroxy tail terminated cyanobiphenyl liquid crystals,” Liquid Crystals, 2019, 46, 397. 10.1080/02678292.2018.1502373
F. S. Fouad, T. Ness, K. Wang, C. E. Ruth, S. Britton, R. J. Twieg, “Biphenylyl-1,2,4-oxadiazole Based Liquid Crystals - Synthesis, Mesomorphism, Effect of Lateral Monofluorination,” Liquid Crystals, 2019, 46, 2281. 10.1080/02678292.2019.1623335
Y. Cao, H. Yu, N. L. Abbott, V. M. Zavala, “Machine Learning Algorithms for Liquid Crystal-Based Sensors,” ACS Sensors, 2018, 3, 2237. 10.1021/acssensors.8b00100
R. Abbasi, C. Wang, Y. Bai, N. L. Abbott, “Phosphorylation Status of Peptide Monolayers Modulates Hydrogen Bonding and Orientations of Nematic Liquid Crystals,” Liquid Crystals, 2018, 45, 2253. 10.1080/02678292.2018.1509389
T. Szilvási†, N. Bao†, H. Yu. R. J. Twieg, M. Mavrikakis, N. L. Abbott, "Redox-Triggered Orientational Responses of Liquid Crystals to Chlorine Gas," Angewandte Chemie International Edition, 2018, 57, 9665. 10.1002/anie.201803194
H. Yu†, T. Szilvási†, P. Rai, R. J. Twieg, M. Mavrikakis, N. L. Abbott, "Computational Chemistry-Guided Design of Selective Chemoresponsive Liquid Crystals Using Pyridine and Pyrimidine Functional Groups," Advanced Functional Materials, 2018, 28, 1703581. 10.1002/adfm.201703581
T. Szilvási†, N. Bao†, H. Yu, R. J. Twieg, M. Mavrikakis, N. L. Abbott, "The Role of Anions in Adsorbate-induced Anchoring Transitions of Liquid Crystals on Surfaces with Discrete Cation Binding Sites. Soft Matter," Soft Matter, 2018, 14, 797. 10.1039/c7sm01981e
K. Nayani, P. Rai, N. Bao, H. Yu, M. Mavrikakis, R. J. Twieg, N. L. Abbott, "Liquid Crystals with Interfacial Ordering that Enhances Responsiveness to Chemical Targets," Advanced Materials, 2018, 30, 1706707. 10.1002/adma.201706707
Y. K. Kim, Y. R. Huang, M. Tsuei, X. Wang, N. C. Gianneschi, N. L. Abbott, "Multi-Scale Responses of Liquid Crystals Triggered by Interfacial Assemblies of Cleavable Homopolymers," ChemPhysChem, 2018, 19, 2037. 10.1002/cphc.201800106
Y. K. Kim, K. R. Raghupathi, J. S. Pendery, P. Khomein, U. Sridhar, J. J. de Pablo, S. Thayumanavan, N. L. Abbott, "Oligomers as Triggers for Responsive Liquid Crystals," Langmuir, 2018, 34, 10092. 10.1021/acs.langmuir.8b01944
Y. K. Kim, X. G. Wang, P. Mondkar, E. Bukusoglu, N. L. Abbott, "Self-reporting and self-regulating liquid crystals," Nature, 2018, 557, 539. 10.1038/s41586-018-0098-y
T. Szilvási, L. T. Roling, H. Yu, P. Rai, S. Choi, R. J. Twieg, M. Mavrikakis, N. L. Abbott, "Design of Chemoresponsive Liquid Crystals through Integration of Computational Chemistry and Experimental Studies," Chemistry of Materials, 2017, 29, 3563. 10.1021/acs.chemmater.6b05430
L. T. Roling, J. Scaranto, J. Herron, H. Yu, S. Choi, N. L. Abbott, M. Mavrikakis, "Towards First-Principles Molecular Design of Novel Liquid Crystals-Based Chemoresponsive Systems," Nature Communications, 2016, 7, 13338. 10.1038/ncomms13338
C. D. Ma, L. Adamiak, D. S. Miller, X. Wang, N. C. Gianneschi, N. L. Abbott, "Liquid Crystal Interfaces Programmed with Enzyme-Responsive Polymers and Surfactants," Small, 2015, 11, 5747. 10.1002/smll.201502137
† Equal contribution
